Electrochemical performance of Nickel foam electrode in Potassium Hydroxide and Sodium Sulfate electrolytes for supercapacitor applications
DOI:
https://doi.org/10.52547/jcc.4.3.3Keywords:
Electrochemical performance, Nickel Foam, Supercapacitor, Electrolyte, Current CollectorAbstract
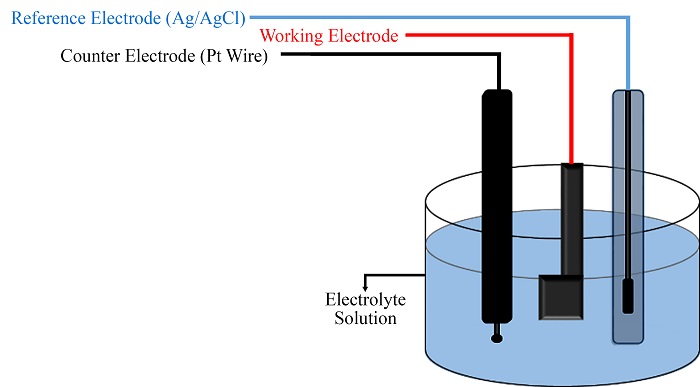
Nickel foam is a shallow-density metal part with very high electrical and thermal conductivity. Nickel foam is used widely as the current collector in electrochemical energy storage. In previous research, the electrolytes used for electrodes in energy sources in the laboratory are often of the aqueous electrolyte type due to their low cost and easy access. In this study, studies were performed to identify and confirm the accuracy of nickel foam, such as scanning electron microscopy and X-ray energy diffraction spectroscopy. Then, in common electrolytes, 1 M potassium hydroxide (KOH) and 0.1 M sodium sulfate (Na2SO4) were investigated by CV, GCD, and EIS analyses. Finally, according to the data and results, the desired electrolyte can be selected depending on the type of use in different environments.
References
E. Niknam, H. Naffakh-Moosavy, S.E. Moosavifard, M.G.J.J.o.A. Afshar, Compounds, Amorphous V-doped Co3S4 yolk-shell hollow spheres derived from metal-organic framework for high-performance asymmetric supercapacitors, 895 (2022) 162720.
E. Niknam, H. Naffakh-Moosavy, S.E. Moosavifard, M.G.J.J.o.E.S. Afshar, Multi-shelled bimetal V-doped Co3O4 hollow spheres derived from metal organic framework for high performance supercapacitors, 44 (2021) 103508.
Q. Wang, Y. Zhang, H. Jiang, C. Meng, In-situ grown manganese silicate from biomass-derived heteroatom-doped porous carbon for supercapacitors with high performance, Journal of Colloid and Interface Science 534 (2019) 142-155 DOI: https://doi.org/10.1016/j.jcis.2018.09.026.
N. Blomquist, T. Wells, B. Andres, J. Bäckström, S. Forsberg, H. Olin, Metal-free supercapacitor with aqueous electrolyte and low-cost carbon materials, Scientific Reports 7(1) (2017) 39836 DOI: 10.1038/srep39836.
C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang, J. Zhang, A review of electrolyte materials and compositions for electrochemical supercapacitors, Chemical Society Reviews 44(21) (2015) 7484-7539.
L. Athouël, F. Moser, R. Dugas, O. Crosnier, D. Bélanger, T. Brousse, Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte, The Journal of Physical Chemistry C 112(18) (2008) 7270-7277.
M. Moradi, F. Hasanvandian, M. Ghahraman Afshar, A. Larimi, F. Khorasheh, E. Niknam, S. Rahman Setayesh, Incorporation of Fe in mixed CoCu-alkoxide hollow sphere for enhancing the electrochemical water oxidation performance, Materials Today Chemistry 22 (2021) 100586 DOI: https://doi.org/10.1016/j.mtchem.2021.100586.
B. Ye, C. Gong, M. Huang, Y. Tu, X. Zheng, L. Fan, J. Lin, J. Wu, Improved performance of a CoTe//AC asymmetric supercapacitor using a redox additive aqueous electrolyte, RSC advances 8(15) (2018) 7997-8006.
L. Zhang, X. Hu, Z. Wang, F. Sun, D.G. Dorrell, A review of supercapacitor modeling, estimation, and applications: A control/management perspective, Renewable and Sustainable Energy Reviews 81 (2018) 1868-1878.
S.Z. Golkhatmi, A. Sedghi, H.N. Miankushki, M. Khalaj, Structural properties and supercapacitive performance evaluation of the nickel oxide/graphene/polypyrrole hybrid ternary nanocomposite in aqueous and organic electrolytes, Energy 214 (2021) 118950.
N.A. Salleh, S. Kheawhom, A.A. Mohamad, Characterizations of nickel mesh and nickel foam current collectors for supercapacitor application, Arabian Journal of Chemistry 13(8) (2020) 6838-6846.
H. Kennaz, A. Harat, O. Guellati, D.Y. Momodu, F. Barzegar, J.K. Dangbegnon, N. Manyala, M. Guerioune, Synthesis and electrochemical investigation of spinel cobalt ferrite magnetic nanoparticles for supercapacitor application, Journal of Solid State Electrochemistry 22(3) (2018) 835-847.
S.Z. Golkhatmi, M. Khalaj, A. Izadpanahi, A. Sedghi, One-step electrodeposition synthesis of high performance Graphene/Cu2O nanocomposite films on copper foils as binder-free supercapacitor electrodes, Solid State Sciences 106 (2020) 106336.
M.I. Abdullah, A. Hameed, N. Zhang, M.H. Islam, M. Ma, B.G. Pollet, Ultrasonically surface-activated nickel foam as a highly efficient monolith electrode for the catalytic oxidation of methanol to formate, ACS Applied Materials and Interfaces 13(26) (2021) 30603-30613.
W. Kong, C. Lu, W. Zhang, J. Pu, Z. Wang, Homogeneous core–shell NiCo 2 S 4 nanostructures supported on nickel foam for supercapacitors, Journal of Materials Chemistry A 3(23) (2015) 12452-12460.
Z. Yu, Z. Cheng, X. Wang, S.X. Dou, X. Kong, High area-specific capacitance of Co (OH) 2/hierarchical nickel/nickel foam supercapacitors and its increase with cycling, Journal of Materials Chemistry A 5(17) (2017) 7968-7978.
W. Xing, S. Qiao, X. Wu, X. Gao, J. Zhou, S. Zhuo, S.B. Hartono, D. Hulicova-Jurcakova, Exaggerated capacitance using electrochemically active nickel foam as current collector in electrochemical measurement, Journal of Power Sources 196(8) (2011) 4123-4127.
Y.-J. Shih, Y.-H. Huang, C.P. Huang, Electrocatalytic ammonia oxidation over a nickel foam electrode: Role of Ni (OH) 2 (s)-NiOOH (s) nanocatalysts, Electrochimica Acta 263 (2018) 261-271.
X. Xiong, D. Ding, D. Chen, G. Waller, Y. Bu, Z. Wang, M. Liu, Three-dimensional ultrathin Ni (OH) 2 nanosheets grown on nickel foam for high-performance supercapacitors, Nano Energy 11 (2015) 154-161.
Y.F. Yuan, X.H. Xia, J.B. Wu, J.L. Yang, Y.B. Chen, S.Y. Guo, Nickel foam-supported porous Ni (OH) 2/NiOOH composite film as advanced pseudocapacitor material, Electrochimica Acta 56(6) (2011) 2627-2632.
R.M. Obodo, N.M. Shinde, U.K. Chime, S. Ezugwu, A.C. Nwanya, I. Ahmad, M. Maaza, P.M. Ejikeme, F.I. Ezema, Recent advances in metal oxide/hydroxide on three-dimensional nickel foam substrate for high performance pseudocapacitive electrodes, Current Opinion in Electrochemistry 21 (2020) 242-249.
B. Pierozynski, T. Mikolajczyk, M. Luba, A. Zolfaghari, Kinetics of oxygen evolution reaction on nickel foam and platinum-modified nickel foam materials in alkaline solution, Journal of Electroanalytical Chemistry 847 (2019) 113194.
G.-W. Yang, C.-L. Xu, H.-L. Li, Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance, Chemical Communications (48) (2008) 6537-6539.
H. Yin, L. Jiang, P. Liu, M. Al-Mamun, Y. Wang, Y.L. Zhong, H. Yang, D. Wang, Z. Tang, H. Zhao, Remarkably enhanced water splitting activity of nickel foam due to simple immersion in a ferric nitrate solution, Nano Research 11(8) (2018) 3959-3971.
C. Fang, D. Zhang, A large areal capacitance structural supercapacitor with a 3D rGO@ MnO 2 foam electrode and polyacrylic acid–Portland cement–KOH electrolyte, Journal of Materials Chemistry A 8(25) (2020) 12586-12593.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 The University of Georgia Publishing House (UGPH)

This work is licensed under a Creative Commons Attribution 4.0 International License.

